pH
value from 0 (sour) to 14 (basic alkaline)
pH value is showing if a liquid is sour or basic alkaline
[web01]. A sour liquid for example is dissolving food
faster or is attacking metals so they become rusty. A
basic liquid is eliminating dirt (like washing powder).
Mostly the liquid is a mixed one, also a chemical solution
[web01].
<pH value depends on the quantity of carbon dioxide
ions (CO2) [this is the sour element] and depends on
hydrogen carbonate ions (HCO3-) [this is the basic
alkaline element].> [web11]
(original in German: <Der pH-Wert
hängt ab von der Menge an Kohlendioxid (CO2)
[das saure Element] und Hydrogenkarbonat-Ionen (HCO3-)
[das basische Element].> [web11])
This scheme is going from 0 to 14 points. pH7 is neutral:
- pH value under 7 (<7) = sour [web16] respectively
this is a sour chemical solution [web18]
- pH value of 7 is neutral (e.g. pure water) [web16]
or a neutral chemical solution [web18]
- pH value over 7 (>7) = alkaline (basic) [web16]
respectively can be a basic alkaline solution [web18].
The item of pH
<The item [pH] is derived from Latin "potentia
hydrogenii" (= power of hydrogen) and is indicating the
hydrogen ion concentration in g per liter. [...] Hydrogen
ion concentration in a liquid indicates if the liquid is
sour, basic alkaline or neutral. [...] When acids or bases
are dissolved in water then they are giving hydrogen ions
by dissociation and are therefore changing pH value.>
[web07]
(original in German: <Der Begriff
[pH] leitet sich vom lateinischen „potentia hydrogenii“
(=die Kraft/Stärke des Wasserstoffs) ab und gibt die
Wasserstoffionenkonzentration in g/Liter an. [...] Die
Wasserstoffionenkonzentration einer Flüssigkeit gibt an,
ob sie sauer, basisch oder neutral ist. [...]
Werden Säuren oder Basen in Wasser gelöst, geben diese
durch Dissoziation Wasserstoffionen ab und verändern
dadurch den pH-Wert.> [web07]
<Scale of pH values is limited by the solubility of
acids and bases in water. With very high or very low pH
values and in concentrated salt solutions not the
concentrations are decisive for the pH value but the
activities of the ions are decisive. The activities do not
depend directly from the ion concentrations.> [web01]
(original in German: <Die pH-Skala wird nur
begrenzt durch die Löslichkeiten von Säuren oder Basen
in Wasser. Bei sehr hohen oder sehr niedrigen pH-Werten
und in konzentrierten Salzlösungen sind nicht die
Konzentrationen für den pH-Wert entscheidend, sondern
die Aktivitäten der Ionen. Aktivitäten sind von den
Ionenkonzentrationen nicht linear abhängig.> [web01]
Responsible for pH value in the body are the lungs (supply
of hydrogen), kidneys and the liver (responsible for
excretion of toxics). [web16,web17]
Technical terms: alkalosis - acidosis
An acidosis is a sour state respectively an
overacidification. An alkalosis is a too basic
alkaline state [web11].
In the blood the pH value is 7.35 to 7.45 [web01]. In
the blood a pH value under 7.37 is rated as an
acidosis and higher as 7.45 is rated as an alkalosis
[web11].
In urine and saliva a pH7 is rated as neutral. There
are more tolerances concerning sour pH values
depending from the author.
pH values in food have an influence to the pH value in the
urine and in saliva. But pH value in the blood is always
buffered between 7.35 and 7.45 when the person is healthy
[web01].
Additionally one has to see the difference how acid or
basic the food is and if they FORM acids or basics - this
is an important DIFFERENCE. So what is important is what
the food is FORMING: forming acids or basics (conclusion
Palomino).
Examples of pH values
Here are some examples of pH values of substances and
food:
Battery acid <1 - gastric acid (sober) 1.0-1.5 - lemon
2.4 - Cola 2.0-3.0 - vinegar 2.5 - juice of acid cherries
(
Prunus cerasus subsp.
acida) 2.7 - orange
and apple juice 3.5 - wine 4.0 sour milk 4.5 - beer
4.5-5.0 - acid rain > 5.0 - coffee 5.0 - tea 5.5
[black] - skin surface of the human 5.5 - rain (natural
precipitation) 5.6 - mineral water [with carbonic acid]
6.0 - milk 6.5 - water (depending on hardness) 6.0-8.5 -
human saliva 6.5-7.4 - pure water 7.0 - blood 7.4 -
seawater 7.5-8.4 - pancreatic juice (intestinal juice) 8.3
- soap 9.0-10.0 - household ammonia 11.5 - bleacher 12.5 -
concrete 12.6 - 13.5-14 sodium hydroxide solution [web01]
The list of some pH values
sodium hydroxide solution 13.5-14
concrete 12.6
bleacher 12.5
household
ammonia 11.5
soap 9.0-10.0
pancreatic juice
(intestinal juice) 8.3
seawater 7.5-8.4
blood 7.4
pure water 7.0
human saliva
6.5-7.4
water
(depending on hardness) 6.0-8.5
milk 6.5
mineral water [with
carbonic acid] 6.0
rain (natural precipitation) 5.6
skin surface of
the human 5.5
tea 5.5
[black]
coffee
5.0
acid
rain > (over) 5.0
beer 4.5-5.0
sour
milk 4.5
wine 4.0
orange
and apple juice 3.5
juice of acid
cherries (Prunus cerasus subsp. acida)
2.7
vinegar 2.5
Cola 2.0-3.0
lemon 2.4
gastric acid
(sober) 1.0-1.5
battery acid <1

Table with pH values [1] [web01]
Here are more
examples of pH values of substances and food from
another source:
Battery acid pH 1, gastric juice pH
1, lemon pH2, dilute hydrochloric acid pH2,
table vinegar pH3, lemonade pH3, sauerkraut
(sourcrout) pH4, Coca-Cola pH4, sour milk pH5,
mineral water [with carbonic acid] pH5, saliva
pH6, milk pH6, distilled water [pure H2O]
pH7, seawater pH8, intestinal juice pH 8, soap
solution pH10, ammonia pH11,
dilute sodium hydroxide solution pH11 [web07].
The values are shown within international
universal pH color scale:
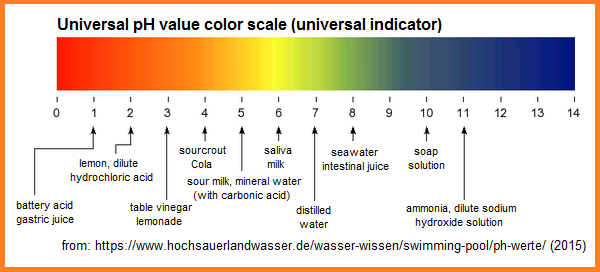
pH values within the international universal color pH
color scale [2] [web07]
Examples
of substances forming acids in the
human body
Be aware that concerning food it's not so important
how acid the food is, but it's important what effect
it has in your body FORMING acids or basics.
Here is food
forming much acid
-- meat, above all pork
-- milk products (cheese, yogurt etc.)
-- white flour products
-- industrially produced pastry products
-- industrially produced noodle products
-- industrial sugar
-- mineral water with carbonic acid
-- lemonades
-- coffee
-- industrially proceeded fruit juices
-- sweets and chocolates
-- instant meals [web18].
Some pH values of the human body
-- normal soap: considerably basic
alkaline [web01]
-- amniotic fluid: pH8,5 [web02]
-- sperm: basic alkaline [web01]
-- arterial blood: pH7,35-7,45
[web01]
-- vagina: sour
[web01]
-- skin of the human body: slightly
sour about pH5.5 [web01]
-- today's washing
lotions (with water, glycerin, sodium chloride,
sodium thiosulfate, sodium hydrogen carbonate,
distearates, tensides): pH5 [web01]
pH value in the human blood is always the same
pH7.35-7.45
[web01] with the best working immune system [web18].
pH values of organs and body liquids
Table: pH values of organs and
body liquids
|
| Body liquid, organ etc. |
normal pH value |
| Blood |
about pH7,4
(pH7,36–7,44; depending from the definition
also 7,37–7,43) |
| Urine |
normally
slightly sour, depending from the food can
also be alkaline (pH4,5–7,9) |
| Stomach |
pH1–4 |
| Bile |
pH6,5–8,2 |
| Stool |
pH7 |
| Saliva |
pH5,5–7,8 |
| Skin |
about pH5,5
("acid mantle") |
| Saliva |
pH4,5
(protection effect against infections by
antibacterial effect) |
| Vagina
(above all the lower 2/3s) |
pH4–5 |
| Secretion
of cervix |
pH7–8,5 |
| Sperm |
pH7–7,8 |
Body
cells
|
Normally there are more
hydrogen ions in the body than in the blood
- body cells have normally a pH value of
7,0–7,3. |
|
[web16]
|
pH value in the urine is variable: it's the
indicator for the food indicating health dangers.
[web05]
pH value of the skin: about pH5.5
<Human skin is slightly sour, pH ≈
5.5. Acid mantle is a protection
from germs.> [web01]
(original in German:
<Die Haut des Menschen ist leicht sauer, pH ≈
5,5. Der Säuremantel ist
ein Schutz vor Krankheitserregern.> [web01])
Body care has to be basic and then
the acid mantle is reinstalling itself again:
original in German:
<Eine naturbalassene, basische
Körperpflege mit leicht basischen Werten
(pH 7,4), wie sie heute von einigen
wenigen Herstellern angeboten wird, ist
sehr viel geeigneter für die tägliche
Hautpflege, da dieser pH-Wert die Haut
nicht austrocknet. [...] Unser Körper ist
- aufgrund der extrem hohen Säurebelastung
- nicht mehr in der Lage, die anfallenden
Säuren über die Nieren oder den Darm
auszuleiten, so dass er die Haut zur Hilfe
nehmen muss. Aus diesem Grund ist der
pH-Wert der Haut auch extrem niedrig. Er
liegt im Durchschnitt bei pH 5,5.>
[web02] |
|
|
Translation:
<A natural basic alkaline body care
with slightly basic pH values (pH7.4) as
it's offered today by some producers is
much more appropriate for a daily skin
care because these pH values are not
drying out the skin. [...] Our body is -
because of the high charge of acids - not
capable to excrete the acids over the
kidneys and intestine so the skin has to
be taken as a help. By this reason the pH
value of the skin is extremely low. The
average is about pH5.5.> [web02]
|
pH value with soaps and lotions
Soaps are basic and provoke a dry skin. After the body
cure the acid mantle is reinstalling itself excreting
acids by the skin again.
original in German:
<Seifen sind deutlich basisch und
„trocknen“ die Haut aus, sie entfernen die
Fettschicht und zerstören die
Säureschicht.> [web01]
<Die
eingelagerten Säuren wollen über die Haut
entweichen, was grundsätzlich am
leichtesten in einem basischen Umfeld
möglich ist. [...] Da Säuren und Basen
immer eine Reaktion eingehen, regt die
basische Hautpflege den Körper an, die
eingelagerten Säuren und
Schlacken aus dem überwiegend sauren
Milieu der Zellen zu lösen und in die
basische Umgebung abzugeben. Auf diese
Weise hilft eine basische Hautpflege dem
Körper effektiv bei seiner Entsäuerung.
[...] Die Talgdrüsen werden angeregt, die
natürliche Rückfettung der Haut zu
aktivieren.> [web02]
|
|
|
Translation:
<Soaps are considerably basic "drying"
out the skin, they delete the fatty layer
and they are destroying the acid
layer.> [web01]
<The
deposed acids want to slip away by the
skin what is basically possible in a
basic alkaline environment [...] As
acids and basics always have a reaction
basic body cure is stimulating the body
to detach the deposed acids and slags
from the sour dominated environment of
the cells giving the slags to the basic
environment. By this a basic body cure
is helping to deacidify the body
effectively. [...] Oil glands are also
stimulated to activate forming of a new
fatty layer.> [web02]
|
Sour soaps and sour detergents are damaging the
skin
The claim that one should protect the "acid mantle" is
WRONG. When sour detergents are applied the body is
only more and more acid because acids cannot slip away
passing the skin any more [web02].
Original in
German:
<Wer über einen langen
Zeitraum eine saure und zu allem
Überfluss auch noch chemisch
hergestellte Körperpflege verwendet,
erzeugt im Körper eine Säureflut, die
enorme körperchemische Wirkungen
hervorruft. Die eingelagerten Säuren
wollen über die Haut entweichen, was
grundsätzlich am leichtesten in einem
basischen Umfeld möglich ist. Wirken
wir diesem Bestreben durch das
Einreiben mit einer sauren
Körperlotion entgegen, werden die
Säuren wieder in die Körpersäfte
zurückgedrängt. Die Folge ist, dass
die Säuren zu einem späteren Zeitpunkt
wieder austreten wollen. Der Anwender
saurer Hautpflegeprodukte hat schnell
das Gefühl, sich wieder reinigen zu
müssen. Daher ist es keine Seltenheit,
dass viele Menschen sogar öfter als
einmal täglich duschen.> [web02]
|
|
|
Translation:
<Persons applying a sour and chemically
produced body care is provoking an acid
tsunami provoking an enormous bodily
chemical effect. Acid deposits want to slip
away by the skin what is principally
possible in a basic environment. When we
block this movement applying sour body
lotions the acids are pressed back into the
body juices. The consequence is that the
acids want to slip away later. The persons
applying sour skin care products have fast
the feeling that they have to wash
themselves again. Therefore it's not rare
that many persons are taking a shower even
several times per day.> [web02]
|
Sour soaps and
detergents are attacking the skin and are
provoking dermatophytes and itching etc.
original in
German:
<Immer häufiger
reagiert die Haut auch allergisch auf eine
chemisch saure Körperpflege. Die
Reaktionen reichen von unangenehmem
Hautjucken über Brennen bis hin zu
teilweise offenen Hautstellen. Ist die
Haut angegriffen, bietet sie eine ideale
Brutstätte für Hautpilze aller Art. Ein
saures Hautmilieu ermöglicht die
Ausbreitung von Genital-, Fuss-,
Nagel- und Hautpilzen. Betrachtet
man das masslose Pflegebedürfnis des
Menschen der Neuzeit, wundert es nicht,
dass die Anzahl der Pilzinfektionen stetig
steigt.> [web02]
|
|
|
Translation:
<Always more skin is also reacting in an
allergic way against chemical sour body
care. The reaction can be from itches to
burning feeling up to open skin. When skin
is attacked these zones are the perfect
breeding places for dermatophytes of all
kind. A sour skin environment is
facilitating the spreading of dermatophytes
in the genital zone, on the feet (athlet's
foot), under the nails (nail fungus) and on
the skin. Considering the cure desire of
humans of our times it's no wonder that the
number of fungal infections is always
rising.> [web02]
|
Fungal infections can only live in a sour environment
- therefore a soap should be basic alkaline:
<Fungal skin infection [...] can only live when a
sour body environment is found.> [web02]
(original in German: <Hautpilz [...]
ist nur dann überlebensfähig, wenn er ein saures
Körpermilieu vorfindet.> [web02])
pH value in the vagina and sperm
<Vagina of a woman is sour for killing germs.
Sperm of the man is basic. Spermium is coming and is
provoking a neutral environment then for the best
movement activity.> [web01]
(original in German:
<Die Scheide der Frau ist sauer, um
Krankheitserreger abzutöten. Das Sperma des Mannes
ist basisch. Das Spermium kommt dann in ein
neutrales Milieu zur optimalen Bewegung.>
[web01])
pH value of water
Here are some pH values of different water types:
-- bath water with a basic bath salt: pH8.5 [web02]
-- Kangen water: basic [web04]
-- bath water without supplements: pH7 neutral
[web02]
-- tap water: pH6.5-9.5 [web01]
-- mineral water with carbonic acid: pH6.0 [web01]
-- bath water after 1 hour of bath: sour [web01]
-- water (depending on hardness) pH6.0-8.5 [web01]
-- pure water [H2O] 7.0 [web01].
<According to drinking water
regulations drinking water from the tap has to be
with a pH value between 6.5 and 9.5. pH value of tap
water can be arranged with chemicals to the desired
pH level.> [web01]
(original in German:
<Gemäß der Trinkwasserverordnung
darf das Trinkwasser aus der
Leitung einen pH-Wert zwischen 6,5 und 9,5
aufweisen. Das Wasser kann mit Chemikalien auf den
gewünschten Wert eingestellt werden.> [web01])
Bath water is becoming sour in the course of the
time:
<When the bathing person is leaving the bath
water after about one hour pH value has sunk
considerably. This is the proof that the bath water
is taking acids which were excreted from the bathing
body reaching the bathing water by slipping away
passing the skin.> [web01]
(original in German:
<Verlässt der Badende
nach ca. einer Stunde Badedauer das Wasser, ist
der pH-Wert messbar gesunken. Damit wird der
Beweis erbracht, dass das Badewasser die Säuren
aufnimmt, die vom Körper ausgeschieden wurden und
über die Haut in das Wasser gelangt sind.>
[web01])